Periodontics: Oral Health and Wellness I. Understanding Periodontal Health, Recognizing Disease States and Choices in Treatment Strategies
Course Number: 50
Course Contents
Regenerative Surgery
Regenerative procedures add the potential to restore lost tissues and attachment to the goals of osseous surgery. When the disease process is halted, or sufficiently slowed, the restored structures could be lasting. The procedures and materials include barriers to prevent the epithelium from growing into the bony defect and to provide an increased opportunity for bone growth within the defect. Other regenerative procedures include osseous grafting utilizing one of a large variety of natural and synthetic materials currently available. Biologically mediated strategies include matrix-derived proteins and/or growth factors.
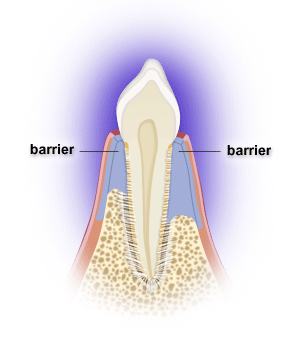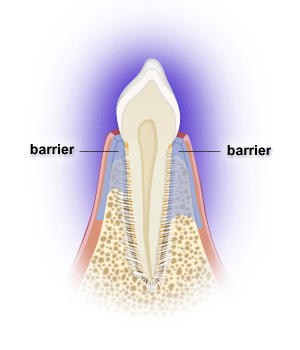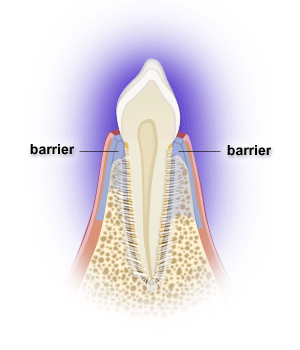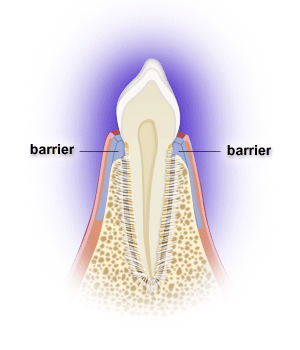
Following periodontal surgery, epithelial, gingival connective tissue, bone and/or periodontal ligament cells may grow along the root surface. Barriers are often used to help guide periodontal ligament cells to grow along the root surface to restore lost tooth support, while preventing the epithelial and gingival connective tissue cells from entering the space. Gains in probing attachment and bone fill may result. The process is called guided tissue regeneration, and one can utilize resorbable or non-resorbable barriers, calcium sulfate, etc. Non-resorbable barriers by their nature last longer, but require removal at a later surgery. Resorbable barriers are used more frequently, but some of them may resorb prior to the length of the time needed for optimal bone fill.
Many osseous grafting materials are currently available. Osseous grafts are generally most predictably successful in treating narrower and deeper 3-walled bony defects. Bony defects with fewer walls have less predictable bone fill results. Barriers can provide containment for grafts as well.
Bone grafting to stimulate bone deposition has been used in periodontal surgery since the 1970's. It involves a surgical procedure to place bone or bone substitute material into a bone defect with the objective of producing new bone and possibly the regeneration of periodontal ligament and cementum. Over time, most of the graft material will be replaced by new bone. In fact, over time all bone is eventually replaced by new bone as illustrated in the bone physiology review.
Autografts utilize the patient's bone, which can be obtained from intra-oral or extra-oral sites. They are the best materials for bone grafting, are very well accepted by the body and may produce the fastest rate of bone growth; however, there is the potential risk of additional discomfort and a secondary procedure. With autografts, the patient is assured of protection from disease transmission and/or immune reaction.
Allografts are obtained from another human source, typically highly processed bone powder from human cadavers. The age and health of the donor can affect the rate of bone regeneration. The risk of disease transmission and/or rejection is handled by processing and quality control. The allografts are freeze-dried at ultra-low temperatures and dried under high vacuum. They are available either demineralized or non-demineralized. Unlike synthetic bone, which only provides scaffolding for osteoconduction, allografts include growth factors which are also osteoinductive. Allografts both induce bone growth and provide an environment that increases the body's regenerative process.
Xenografts are obtained from animal sources; usually cows and/or pigs. They include processed animal bone or growth proteins. Again, the risk of disease transmission and/or rejection is reduced by processing. A synthetic cell-binding peptide (P-15) combined with anorganic bovine-derived hydroxyapatite bone matrix (ABM) mimics autogenous bone and has been proven superior to grafts from some other sources. Like autogenous bone, ABM/P-15 is made of organic and inorganic components. The inorganic component in humans is calcium phosphate. ABM/P-15 contains identical calcium phosphate from a bovine source. The organic component of autogenous bone is collagen, a fibrous tissue which is important for cell binding and proliferation. The collagen component responsible for this function is P-15, which is synthetically replicated it and irreversibly attached it to the inorganic component. The processing at very high temperatures removes the risk of disease transmission or immune reaction. ABM/P-15 was evaluated in 31 patients and found to have promise as a bone replacement graft in 1, 2 and 3-wall bony defects along with open flap mechanical debridement, followed by 3-month recalls for three years.
Synthetic bone grafting materials carry no risk of disease transmission or immune system rejection. They help create an environment that facilitates the body's regenerative process. Examples of synthetic materials include natural and synthetic hydroxyapatites, ceramics, calcium carbonate (natural coral), silicon-containing glasses, and synthetic polymers.
Biologically mediated strategies include materials, such as enamel matrix proteins, that can be pre-mixed with vehicle solution. They are intended as an adjunct to periodontal surgery for topical application onto exposed root surfaces or bone to treat intrabony defects and furcations due to moderate of severe periodontitis. Enamel matrix protein leaves only a resorbable protein matrix on the root surface which makes bone more likely to regenerate. These bone morphogenetic proteins help to initiate a cascade of events leading to the differentiation of progenitor cells into phenotypes involved in periodontal regeneration.
Growth factors and various extracellular matrix (ECM) proteins in bone regulate cell attachment. These proteins provide the osteogenic capacity observed with autografts and allografts. That is, these components provide the chemical cues to initiate and maintain those processes associated with bone formation. Synthetic bone graft materials, whether polymer, ceramic, etc., provide only an inherent osteoconductive capacity. They provide only a physical platform to support cell attachment and tissue development. By incorporating such biological growth factor components within synthetic bone scaffolds a closer imitation of human bone graft material is achieved. Thus a synthetic bone graft can be achieved with the inherent osteogenic properties of an allograft, without the immunological rejection or disease threat potential, and with the inherent osteogenic properties of autograft, without the complications often associated with the graft donor site.