A Clinical Trial To Assess Subgingival Penetration And Retention Of Stannous Fluoride
Reference: M. Klukowska,* D. Ramsey,* C. Combs,* U. Rattanaudompol,* C. Haven,* D. McClenathan,* N. Ramji,* J. Milleman,+ K. Milleman.+ *Procter & Gamble, Cincinnati, OH, USA; +Salus Research Inc., Ft. Wayne, IN, USA.
CLINICAL COMMENT
Gingivitis is a common condition resulting in gingival bleeding and inflammation. Randomized controlled clinical trials show stabilized stannous fluoride dentifrice reduces gingivitis and plaque,1-4 the primary causative agent of gingivitis. Recent learnings show the gingival health benefits of stabilized stannous fluoride dentifrice are due in part to its effect on plaque virulence.5-7 Specifically, stannous fluoride binds to bacterial endotoxins in the gingival sulcus that trigger the inflammatory process, resulting in less toxic plaque and decreased inflammation and bleeding. Findings from this clinical trial provide further evidence that stannous fluoride penetrates subgingivally, reaching sites up to 4mm, and is retained at significant levels for 12 hours. Based on these findings, dental professionals should consider recommending a stabilized stannous fluoride dentifrice, like Crest Gum Detoxify, to treat and prevent gingivitis.
KEY CLINICAL FINDINGS
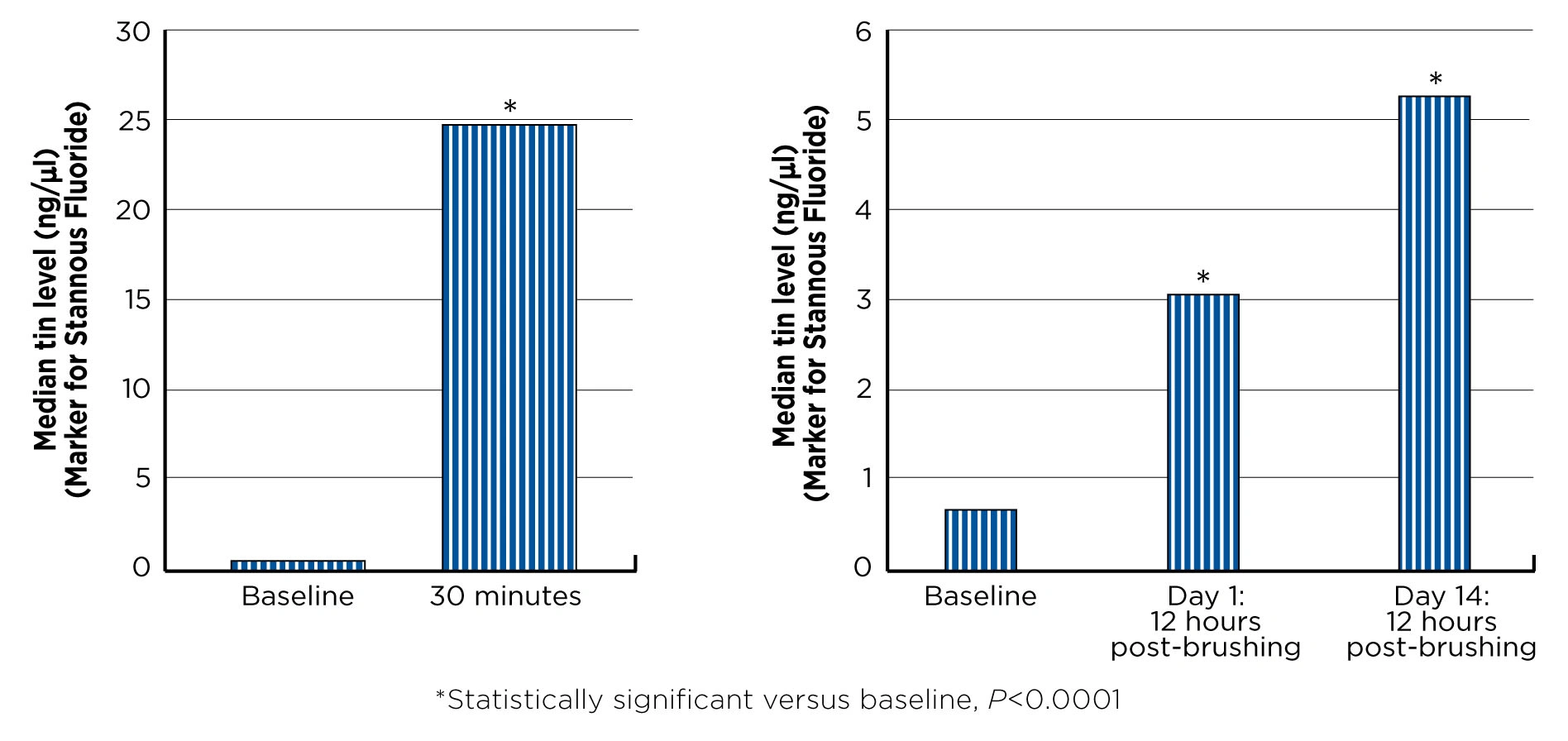
Significant levels of tin, a marker for stannous fluoride, were detected in gingival crevicular fluid (GCF) 30 minutes after brushing at sampling sites of 2–4 mm. The median tin level in GCF was 24.59 ng/μl, which was highly significant versus baseline (P<0.0001). See Figure 1.
Tin levels sampled in GCF 12 hours after brushing on Day 1 and Day 14 were also highly significant versus Baseline (P<0.0001), showing an increasing trend with continued use. See Figure 2.
Figure 1. Significant levels of tin were measured in GCF 30 minutes after brushing versus Baseline.Figure 2. Significant levels of tin were measured in GCF 12 hours post-brushing at Day 1 and Day 14 versus baseline.
Figure 1. Significant levels of tin were measured in GCF 30 minutes after brushing versus Baseline.Figure 2. Significant levels of tin were measured in GCF 12 hours post-brushing at Day 1 and Day 14 versus baseline.
OBJECTIVE
To evaluate stannous fluoride penetration and retention in GCF.
METHODS
This was a controlled, single site study. 23 subjects with at least 20 dental pockets 2 to 4mm with bleeding, and who had not used a stannous fluoride dentifrice in the last 3 months, were enrolled.
After a 2-week washout period in which subjects used a regular sodium fluoride toothpaste (OralB® Cavity Protection) and a soft manual toothbrush (Oral-B
®Indicator), subjects returned for a baseline visit. They were instructed to refrain from brushing after 11pm the night before the baseline visit and to refrain from eating, drinking, chewing gum or tobacco use for 4 hours prior to the visit. GCF samples were taken from up to 10 sites identified as sampling sites.
Subjects were then given a stannous fluoride dentifrice and soft manual toothbrush (Oral-B® Indicator) and asked to brush for one minute. Thirty minutes after brushing, GCF was re-sampled.
Subjects continued using the stannous fluoride dentifrice and soft manual toothbrush at home, twice daily for 2 weeks, in place of their usual hygiene products. At Days 1 and 14, subjects returned to the site, having refrained from brushing and performing any other oral hygiene procedures 12 hours prior to the visit and from eating, drinking, chewing gum or tobacco use for 4 hours prior to the visit. Pre-brushing GCF samples were taken. Twenty subjects completed the trial.
The samples were analyzed by ICP-MS (inductively coupled plasma mass spectrometry).
A Wilcoxon signed-rank test was performed to determine the difference between post-baseline visits and baseline. Statistical tests were 2-sided using a 5% significance level.
1. Mankodi S et al. J Clin Periodontol 2005;32:75-80. 2. Mallatt M et al. J Clin Periodontol 2007;34(9):762-7. 3. Archila L et al. J Periodontol 004;75(12):1592-1599. 4. He T et al. Am J Dent 2013;26:175-179. 5. Klukowska M et al. J Clin Dent 2017;28:16–26. 6. Haught C et al. Am J Dent 2016;29:328-332. 7. Haught C et al. J Clin Dent 2016;27:84–89.