Re-examining the Plaque-Gingivitis Connection and the Role of Stannous Fluoride
Course Number: 579
Course Contents
Clinical Testing is Congruent with In Vitro Findings
Controlled in vivo trials are an important means of confirming the validity and application of laboratory testing. Randomized controlled clinical trials with additional toxicity measurements have confirmed these effects.
Research by Klukowska and colleagues incorporated subgingival plaque sampling in sites up to 4 mm in depth in a 4-week randomized controlled clinical trial of twice daily unsupervised brushing with a 0.454% bioavailable SnF2 dentifrice, wherein both a low gingival bleeding cohort (‘healthy’) and a high bleeding cohort (‘diseased’) were evaluated. 36 Clinical effectiveness trials of marketed dentifrices do not commonly include subgingival plaque sampling, but its inclusion in this trial provided insight into the depths of penetration of SnF2, its retention, and its ability to reduce subgingival plaque toxicity. At Week 4, both cohorts saw significant (42% to 53%) mean reductions in gingival bleeding. The plaque sampling results in both the healthy and diseased groups provided evidence following use of SnF2 of notably decreased LPS/LTA dye activity and TLR activity. Morning wake-up plaque samples via salivary lavage showed significantly suppressed short-chain carboxylic acid toxins for both the low and high bleeding groups as well, suggesting robust substantivity.37,38 By measuring the endotoxin content of the subgingival plaque samples via dye assays and plaque isolates activated gene expression in the TLR reporter cell lines, it was concluded that "SnF2 dentifrice treatment was associated with broad scale reductions in endotoxin content and virulence potentiation properties of dental plaque samples collected subgingivally from patients."39
The researchers noted the important implication of this research and a previous complementary trial:40 The effects of SnF2 to bind with endotoxins and thereby limit TLR4/TLR2 in initiating the inflammatory cascade manifested both in the diseased, high bleeding sites and also in the low bleeding sites with minimal measurable disease, suggesting a preventive as well as a treatment gingivitis strategy.
A subsequent clinical trial evaluating SnF2 penetration within the sulcus and retention in gingival crevicular fluid (GCF) provided further evidence that SnF2 can influence the pathogenicity of microflora subgingivally.41 In this 2-week trial of subjects with a minimum of twenty bleeding dental pockets up to 4mm in depth and no recent SnF2 exposure, GCF samples were analyzed by mass spectrometry for the presence of tin (a stannous fluoride marker) at both 30 minutes and 12 hours after brushing with a bioavailable SnF2 dentifrice on Day 1. The results showed that significant (P<0.0001) levels of tin compared with baseline were detected in the GCF samples. Higher tin levels were seen at Day 14 after 2 weeks of home dentifrice use, suggesting an incremental effect with ongoing use.
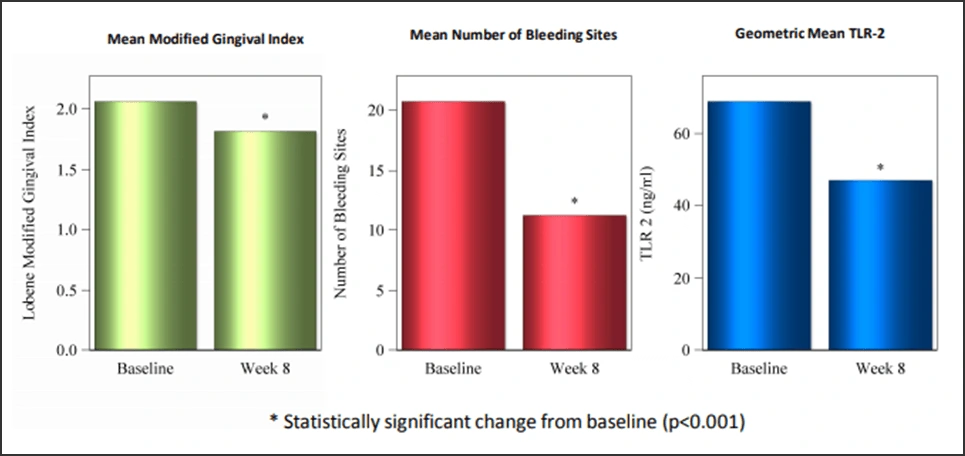
More confirmation of bioavailable SnF2’s ability to diminish the virulence of subgingival plaque – and thus the development of gingivitis – was demonstrated by clinical research evaluating gingival inflammation and bleeding in 99 adult subjects with gingivitis.42 After 8 weeks of at-home 0.454% SnF2 dentifrice use, significant reductions in gingivitis and bleeding versus baseline were observed. These clinical observations were consistent with the results of subgingival plaque sampling, where TLR2 assay analyses of hTLR2 reporter gene activity showed significant (P=0.0004) mean reductions following two months of SnF2 brushing (Figure 9).
Figure 9.
An 8-week clinical trial of 99 subjects with pre-existing disease showed significant reductions in bleeding and gingivitis with bioavailable SnF2, consistent with significant reductions in hTLR2 reporter gene activity via subgingival plaque sampling.42
Fine and colleagues evaluated the clinical effects on gingivitis and the oral microbiome of an SnF2 dentifrice stabilized with zinc phosphate in a controlled trial. Compared to a negative control, results showed significant improvement in bleeding on probing for SnF2 users, coupled with significant reductions in GCF levels of inflammatory markers and gram-negative bacteria.43
Incorporating SnF2 in a dentifrice to yield maximum esthetics and efficacy – including full bioavailability – mandates precise, well-skilled formulation.44,45 In recent years, several technological advances resulting from ongoing scientific innovations and testing have led to bioavailable SnF2 formulations which have provided superior tartar control and whitening benefits, along with the therapeutic benefits, versus a variety of dentifrice controls in multiple clinical trials. The extensive clinical research program by Procter & Gamble on SnF2 dentifrice, which has spanned numerous decades, resulted in a Crest dentifrice being the first to be recognized for seven attributes applicable to toothpastes in the American Dental Association Seal of Acceptance program:46
Prevent or reduce enamel erosion
Prevent cavities
Prevent and reduce plaque
Prevent and reduce gingivitis
Reduce tooth sensitivity
Reduce bad breath
Remove tooth surface stain.
The benefits have been demonstrated in clinical research. In one randomized clinical trial, the novel SnF2 dentifrice demonstrated significantly greater plaque reduction than a negative control and significantly greater tin retention subgingivally than a positive control SnF2 dentifrice.47 In a separate 12-week clinical trial, the novel SnF2 dentifrice produced statistically significant gingival bleeding reductions versus the negative control as quickly as after one week, demonstrating rapid activity. At Week 12, subjects using the SnF2 dentifrice had 33.4% fewer bleeding sites and 6 times greater odds of transitioning from localized or generalized gingivitis (>10% bleeding sites) to generally healthy (<10% bleeding sites) versus the negative control.48 A 2019 meta-analysis of 18 clinical trials evaluating the gingival health effects of bioavailable gluconate chelated SnF2 dentifrices when used for < three months concluded that regardless of baseline level of disease, they significantly reduced gingival bleeding compared to positive and negative controls.49